癌症是免疫病?关键是微环境?美国在研的胰腺癌基金高达372项,全力冲刺这些关键突破点(2024)
时间:2024-10-21 18:00:39 热度:37.1℃ 作者:网络
胰腺癌(Pancreatic Cancer),是一种起源于胰腺的恶性肿瘤,其特征在于早期症状不明显,导致大多数患者在诊断时已处于晚期。胰腺位于腹部深处,主要负责分泌助消化的酶和调节血糖的激素。
尽管近年来在胰腺癌的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
早期诊断难度:由于缺乏特异性早期症状和有效的筛查手段,胰腺癌常常在晚期才被发现。开发早期诊断技术是目前研究的重点之一。
-
治疗选择有限:胰腺癌对现有的化疗和放疗反应较差,手术切除仍是唯一可能治愈的方法,但多数患者在确诊时已无法进行手术。
-
预后不佳:胰腺癌的五年生存率极低,这与其晚期诊断和治疗反应差密切相关。
-
个体化治疗:由于患者间的生物标志物和肿瘤遗传特征差异,需要更多研究以实现更有效的个体化治疗方案。
总体而言,胰腺癌的研究依然面临多方面的挑战,其中最重要的是如何实现早期诊断和改善患者的生存率。未来的研究需要在早期检测技术、新的治疗方法和个体化医疗策略上取得突破。
我们仅对美国国立卫生研究院(NIH)资助的在研胰腺癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Pancreatic Cancer”为检索词、在题目中进行检索,美国NIH针对胰腺癌的在研有372项。
一,谁获得了这些研究?
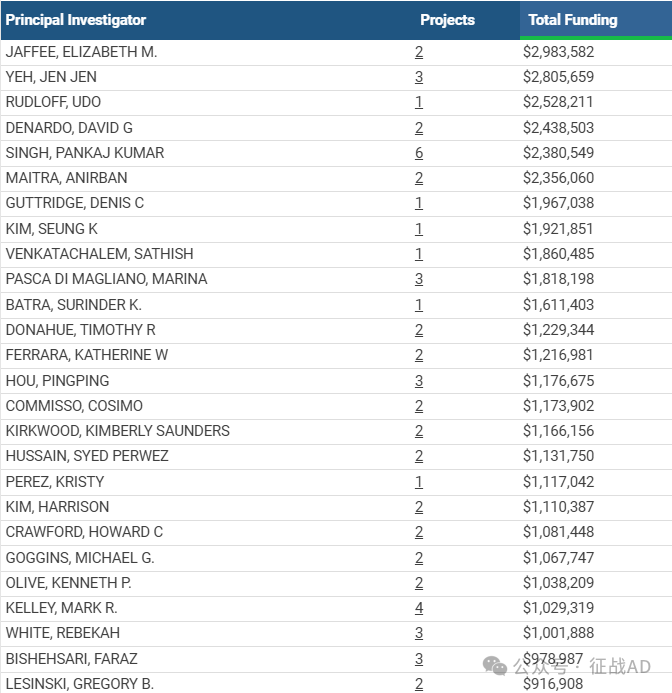
1,在研胰腺癌基金最多的PI
-
JOHNS HOPKINS UNIVERSITY 的 JAFFEE, ELIZABETH M.
-
UNIV OF NORTH CAROLINA CHAPEL HILL 的 YEH, JEN JEN
-
DIVISION OF BASIC SCIENCES - NCI 的 RUDLOFF, UDO
-
WASHINGTON UNIVERSITY 的 DENARDO, DAVID G
-
UNIVERSITY OF OKLAHOMA HLTH SCIENCES CTR 的 SINGH, PANKAJ KUMAR
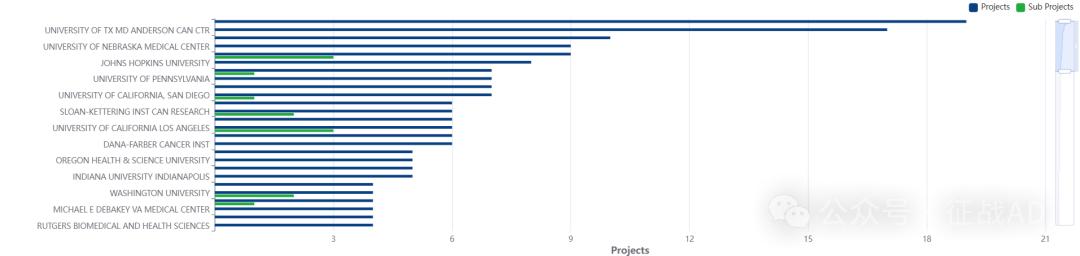
2,胰腺癌基金最多的研究机构
-
德克萨斯大学 MD 安德森癌症中心
-
密歇根大学安娜堡分校
-
约翰霍普金斯大学
-
内布拉斯加大学医学中心
-
北卡罗来纳大学教堂山分校等
二,胰腺癌研究热点是什么?
胰腺癌研究领域总览(根据关键词)
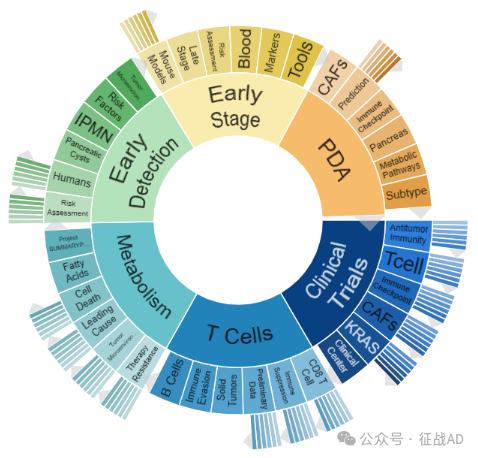
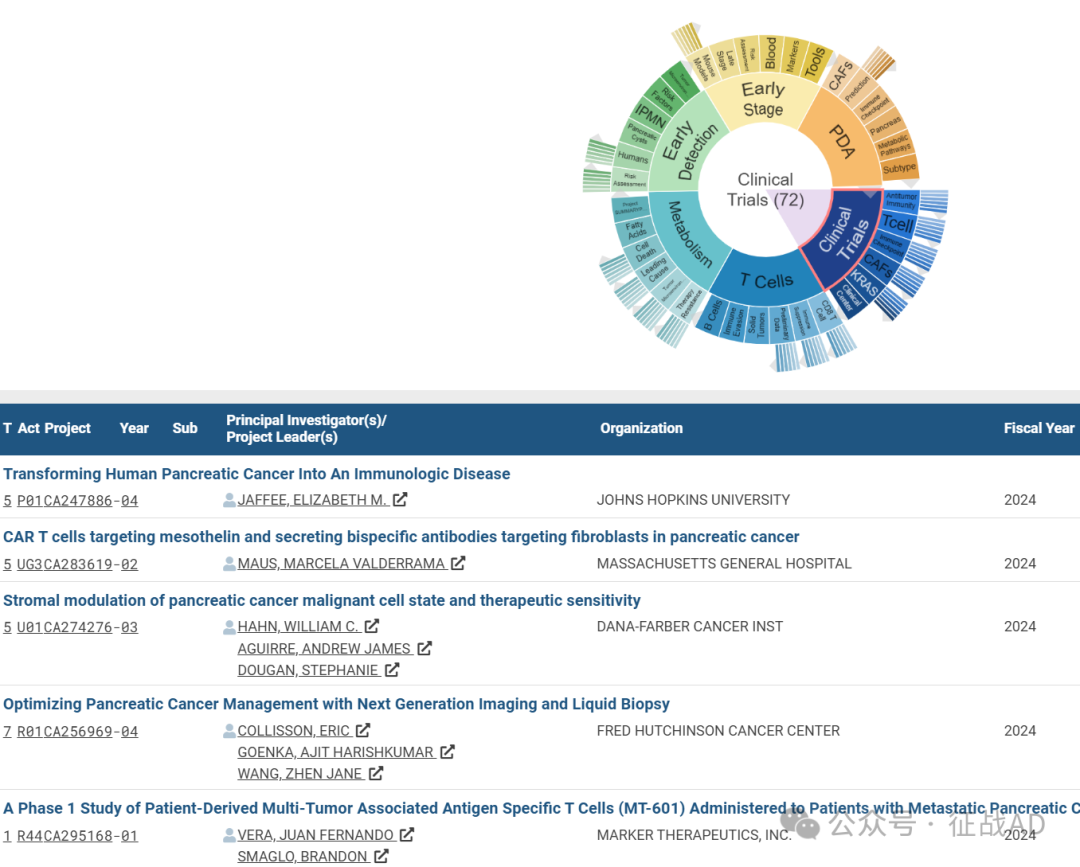
A,关于临床试验(Clinical Trials)的研究项目最多
有 72 项在研基金涉及到了临床试验,关注最多的方面包括抗肿瘤免疫(Antitumor Immunity)、T细胞(T Cell)、免疫检查点(Immune Checkpoint)、临床中心(Clinical Center)、CAFs、KRAS、等研究。
B,关于代谢(Metabolism)的研究项目
有 67 项在研基金涉及到了代谢,关注最多的方面包括项目摘要(Project SUMMARY Pancreatic)、脂肪酸(Fatty Acids)、细胞死亡(Cell Death)、主要原因(Leading Cause)、肿瘤微环境(Tumor Microenvironment)、治疗抵抗(Therapy Resistance)等研究。
C,关于T细胞(T Cell)的研究项目
有 59 项在研基金涉及到了T细胞,关注最多的方面包括CD8 T细胞(CD8 T Cell)、免疫抑制(Immune Suppression)、初步数据(Preliminary Data)、实体瘤(Solid Tumors)、免疫逃避(Immune Evasion)、B细胞(B Cells)等研究。
其他胰腺癌研究大的方向也包括早期检测(Early Detection)、早期阶段(Early Stage)、PDA等。
三,借鉴与突破
我们也分享在胰腺癌领域的几项课题摘要,希望对同仁们有所启发。
A,Transforming Human Pancreatic Cancer Into An Immunologic Disease
Our Team will address two critical problems: 1) inefficient generation of high quality T cells targeted against PDA antigens capable of tumor trafficking and killing; and 2) multiple cellular barriers that comprise stromal and myeloid cells that inhibit effector T cell trafficking and function in the PDA tumor microenvironment (TME). Both clinical studies (“science in patients”) and pre-clinical studies (mouse models) will be conducted to address these issues, and to evaluate novel combinatorial therapies that successfully modulate PDA stroma and chronic inflammation to facilitate improved tumor infiltration of high quality and durable cancer targeted T cells. This program is composed of 4 Projects and 4 Cores.
The four projects will address the common overarching theme that PDA is composed of multiple cell types and signals that inhibit T cell induction, trafficking into, and function in tumors. Each project will address either the induction of quality T cells or the modulation of suppressive cell populations as major barriers to T cell infiltration and activation, and all will integrate agents that bypass these suppressive mechanisms with optimal T cell therapies. Projects 1, 2, and 4 will combine ongoing preclinical studies aimed at uncovering mechanisms of suppression of different barriers with translational clinical trials that study combination therapy to bypass these suppressive mechanisms. Project 3 will conduct a biomarker heavy clinical trial using a multi-arm Platform design that will add and delete immune modulatory arms based on data from biomarker analysis in this Project and from data that feeds into this Project from the other 3 Projects. Standard procedures will be used across Projects to collect and bank serial biospecimens obtained from patients treated on the clinical trials. The Cores will be critical for conducting the proposed assays and for analysis and integration of the data. A Program database will be developed to allow for integration of data generated from these assays across the entire Program. This will be a unique database that will also bring in data from other sources such as the TCGA database, and will provide the Program Team with the ability to compare results based on the genetics and inflammatory composition of each patient’s tumor and their response to the therapy they received.
The final outcomes will include results from a number of therapeutic interventions, approaches to optimize each therapeutic, the potential to further integrate therapies that were tested in one or more projects in future trials, and the ability to develop TME signatures that may further stratify patients for therapeutic interventions.
B, The role of the macroenvironment in pancreatic cancer-induced cachexia
Our hypothesize is that the NF-κB/IL-6/STAT3 signaling axis is a central regulator of the macroenvironment in PDAC-induced cachexia. This hypothesis will be tested in 3 Projects under the support of 4 Cores.Project 1 focuses on the IL-6/STAT3 portion of the NF- κB/IL-6/STAT3 signaling axis. Specifically, studies will define the participation of IL-6 and the IL-6 receptor acting through STAT3 to mediate fat and muscle loss. Project 2 focuses on NF-B within the signaling axis, exploring two fresh concepts in how NF-κB functions in muscle stem cells to promote local muscle inflammation and in PDAC progression by modulating the surveillance of innate and adaptive immune cells. Project 3 will explore the IL-6/STAT3 axis within stroma fibroblasts. Specifically, how STAT3 signaling in stromal mesenchymal cells in the tumor and peripheral tissues produces an immunosuppressive macroenvironment that favors PDAC progression and cancer cachexia. Core A (Administration) will provide administrative structure for the organization of the program. Core B (Human Biospecimens) will maintain a repository of tissues from PDAC patients with and without cachexia and support next generation sequencing analysis. Core C (Immunophenotyping) will provide expertise in scRNA-Seq and multispectral imaging of the tumor, skeletal muscle, and fat in PDAC-induced cachexia. Core D (Biostatistics) will provide biostatistical support.


